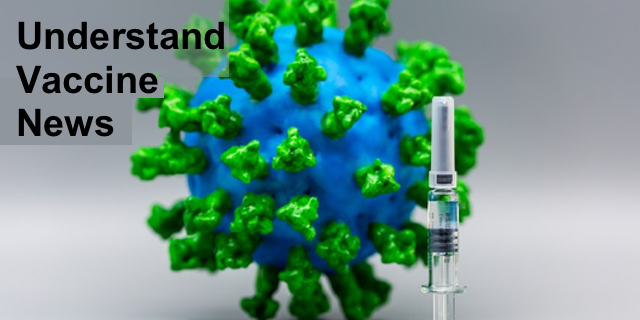
The protrusions on the Coronavirus are a group of three “S” proteins also call “Spike” proteins. These proteins bind to ACE2 receptors on the membranes of cells in the body. This has been a target of many of the over 120 vaccines that have been in production since the pandemic began. A recent intermediary report on one of the vaccines made the news (and the New England Journal of Medicine-NEJM) this week.
The vaccine is made of modified messenger RNA (mRNA) for the protein spike prior to its binding or “fusion” with ACE2 receptors. If you are confused, don’t worry. We’ve laid the groundwork for you to understand this last sentence in several past posts.
What is mRNA? A description of mRNA and the process of transcription in human cells as well as a video can be found here. Viruses use RNA, not DNA, to carry the code for proteins. But the production of proteins is the same. So when the coronavirus COVID-19 connects to the cells lining the nose and its S-proteins fuse to the ACE2 receptors, the process allows the virus RNA into the cells of the nose. There, the virus replicates, making more viruses, each with the S-proteins on the surface. The cell releases all the newly formed viruses.
The vaccine has been created to get the human immune system to recognize the S-proteins prior to fusion. The S-proteins are encapsulated in a nanoparticle. The process of making a nanoparticle is described in the post titled Nanodrugs.
The interim report on the vaccine’s clinical trial describes the development of antibodies in the bodies of participants who were injected with three vaccine types, one that was 25-μg, 100-μg and 250-μg doses.1 The participants were health adults (18-55 years of age). They received two doses of the vaccine 28 days apart. The trial was a safety trial but also an efficacy trial. To determine efficacy, they compared the reactions of their blood to the COVID-19 virus in a petri dish at various times after their first and second injections. Their blood reactions were also compared to the blood serum of a person who had survived COVID-19. Scheduled visits to the doctor scheduled 7 and 14 days after each vaccination and on days 57, 119, 209, and 394. This report is from data up to day 57.
Of those who took the vaccines, by day 43 there were enough antibodies generated against the COVID-19 virus to reduce its infectivity by 80% or more. In other words, the antibodies were enough to reduce the COVID-19 virus to enter cells in these people, replicate and produce other viruses by 80% or more.
The side effects after the first vaccine were rated mild to moderate but were worse for the highest (250-μg) doses. At this point, there is a phase 2 trial going on with 600 healthy adults using 50 μg and 100 μg doses.
Vaccines are important to keeping us all safe. Enjoy this exciting news!
1 https://www.nejm.org/doi/full/10.1056/NEJMoa2022483
Image by pearson0612 from Pixabay