New: Thanks to InVideo, we have a video version of the post below. Check it out at bottom of the post.
Incredible immunotherapy responses in some cancers, but not in others, have puzzled the research community. What is it about some cancers that allows the immune system to be activated? Why are some cancers able to evade the immune system even when immunotherapy is used?
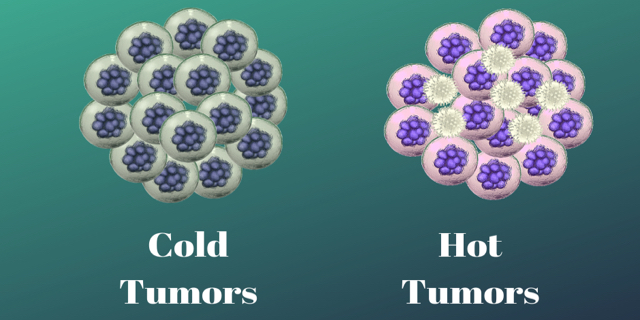
Cold Tumors versus Hot Tumors
Cold Tumors
Researchers call cold tumors an “immune desert.” Cold tumors are tumors that contain few if any infiltrating T cells. The T cells may be surrounding the periphery of the tumor but there isn’t anything to turn on within the tumor itself. One reason is that in cold tumors, there are two types of cells that are part of the immune system but are used by the tumor to keep the good T cells at bay. These turned on immune cells: regulatory T cells or T regs and myeloid cells, called myeloid-derived suppressor cells (MDSC), are immunosuppressors, meaning they turn off the infiltrating T-cells by sending out chemicals like cytokines. One example, pancreatic cancer, has MDSCs–immature macrophages, granulocytes and dendritic cells–the pancreatic cancer cells send out a protein called CXCL1 that messages MDSCs. These MDSCs arrive in droves and suppress T-cell activity. A non-T-cell microenvironment is set up which negatively impacts immunotherapy.
Pancreatic cancer, glioblastomas, ovarian and most prostate, breast, and microsatellite stable (MSS) colorectal cancers are cold.
Hot Tumors
Hot tumors have many infiltrating T-cells and antigens, so the immune system recognizes them and can be easily turned on or stimulated with immunotherapy. Many melanoma, bladder, head and neck cancers and many non-small cell lung cancers are hot. But even some of these hot tumors do not respond to immunotherapy.
Turning Cold Tumors Hot
Turning a cold tumor hot is key. Checkpoint inhibitors, part of the immunotherapy arsenal, turn on T-cells in the tumor. But they have to have a T-cell to turn on. One direction research is taking is identifying the type of infiltrating T-cell that needs to be involved to kill the cancer. In work with mice, researchers have identified CD8-positive T cells as the type of cells that are needed to take out tumors.
Vaccinations are being used to make cold tumors hot. In a small clinical trial with melanoma patients, doctors injected the patients’ tumors with a type of herpes virus that infects cancer cells. The infection made the tumor visible to the correct T cells. The patients were also given a checkpoint inhibitor and patients’ tumors shrank and some patients had a “complete response.”
Recently published research on mice and in phase 1 clinical trials on HER2 metastatic breast cancer involved a vaccination that would stimulate CD8-positive T cells. The trial had positive results and the researchers are going to add checkpoint inhibitors to the next set of trials.
NeoVax
NeoVax is a personalized vaccine that has been used in clinical trials with melanoma patients. Cancers create “neoantigens”  and in the clinical trials, as many as 20 neoantigens from each participant’s tumors were used in the vaccines. The neoantigens are very different from normal cells antigens.
and in the clinical trials, as many as 20 neoantigens from each participant’s tumors were used in the vaccines. The neoantigens are very different from normal cells antigens.
Creating this vaccine is possible because of genetic testing. Patient tumor DNA and normal DNA are sequenced to figure out the mutations that are present in the tumor. Then RNA is sequenced to find the mutations that make the neoantigen proteins.
After identifying the neoantigens, researchers have to figure out which neoantigens were most likely to work. One key to the process has to do with how T cells work. T cells are presented with “other” (non-self) proteins by HLA molecules of the immune system. In order to create these vaccines, recently developed predictive computer algorithms, like one known as NetMHC, are used to see which of the neoantigen proteins will bind to the HLA molecules.
After this, the neoantigens are mixed with a chemical that increases the immune response. The vaccines are used to “train” the participant’s immune system to attack and destroy their cancer. Using as many as 20 neoantigens is a way to target the very heterogeneous tumors. Additionally, this vaccine strategy is good at avoiding the mistake of having immune cells targeting normal cells.
In the most recent trial, researchers found that all the participants had T cells CD8+ killer cells and CD4+ helper cells activated. Complete responses occurred.
First steps for patients
For people with cold cancers like colorectal cancer, the advice is to have molecular testing performed on your tumors. For people with colorectal cancer testing should be done for microsatellite stability, BRAF, KRAS, NRAS, RAS, and HER2 to inform treatment decision making.
Looking for clinical trials
As Dr. Tom Marsilje wrote in his blog, Adventures in living terminally optimistically,
“Most Stage IV patients will eventually reach a point where they will be considering clinical trials and not having information on the science of experimental trial drug classes, written in easily understood ways, is a real issue. Assuming they even know that the government keeps an organized database of clinical trials, here is the“www.clinicaltrials.gov” hit list for “colon cancer” – tonight as I wrote this post, there were 3025 hits.”
Even a scientist like Tom was overwhelmed with the number of clinical trials. To help patients like himself, Tom created a patient curated trial finder for our partner, Fight Colorectal Cancer, in 2017.
And of course, there is Medivizor. Medivizor provides a free service to connect patients to clinical trials. You just sign up.
As promised, here’s a video of post above, thanks to InVideo:






I am very interested in any trials available to me. I am MSS colorectal cancer