A miracle of biochemical interactions, working in a synchronicity that amazes—this is the human body. The human body consists of over 30,000,000,000,000 (30 trillion) cells. Biochemical cycles—directed by proteins built through RNA transcribed/decoded from DNA—run our bodies so that we can eat, drink, run, walk, think and make merry. It happens every second of every day in each cell without our ever considering how it all happens.
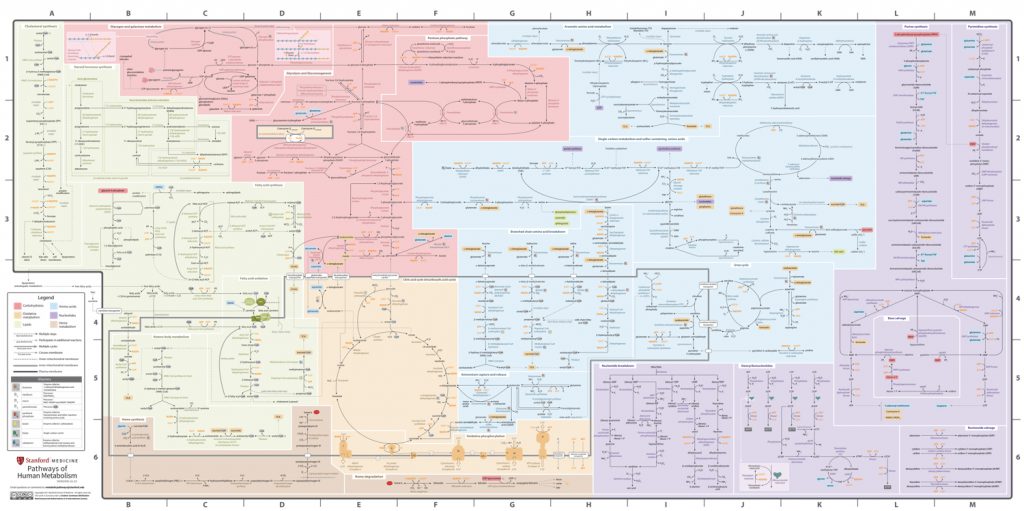
Here is a chart that shows all the biochemical pathways of human metabolism. This tiny picture is actually an enormous, detailed poster. To read it easily, it must be expanded to over 5 feet long by 3 feet wide. That’s complicated!
Proteins
Each of these pathways requires proteins to make them happen. The information required to produce the proteins our bodies need to live has been encoded in nucleic acids by our DNA.
The process of making proteins
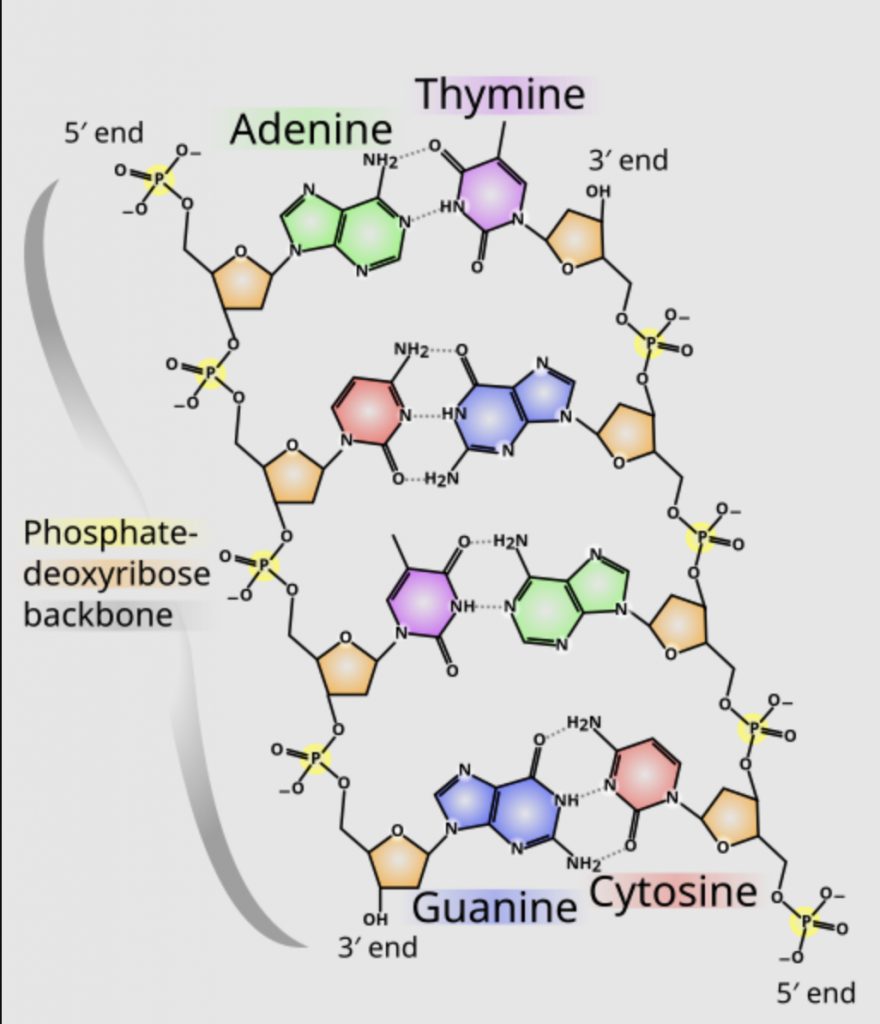
DNA is made up of two strands of phosphate deoxyribose a type of sugar. Each of the phosphate sugars has four types of nucleic acids attached to them: adenine, guanine, cytosine and thymine. The backbone strands link up via the nucleic acids. Adenine joins with thymine on the opposite strand, guanine joins to cytosine, and the sequence of the nucleic acids is the code for the proteins made by our body.
RNA uses the same nucleic acids, except uracil replaces thymine.
Inside the cell
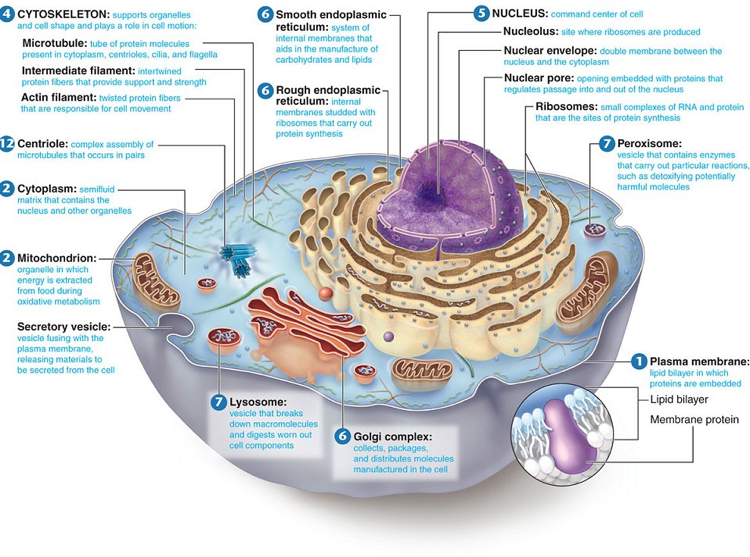
In the nucleus, segments of the DNA encode messenger RNA (mRNA) which travels out of the nucleus to the rough endoplasmic reticulum (see image above). This wavy membrane structure is covered in ribosomes, small protein factories. The mRNA attaches to the ribosomes and transfer RNA (tRNA) brings amino acids that are in the cytoplasm of the cell to the ribosomes.
Amino acids are the building blocks of proteins. The mRNA provides the information as to which amino acid is needed and where it is needed. For example, if the mRNA has a group of three nucleotides (called a codon) like CAA—cytosine, adenine, adenine—the tRNA brings the amino acid glutamine to the ribosome. There it is attached. Then another codon on the mRNA is read and a tRNA will then bring the amino acid corresponding to the next three nucleotides. This process goes on until the ribosome reads a stop message of three nucleotides: uracil, adenine, adenine or uracil adenine, guanine or uracil, guanine, adenine. These tell the ribosome that the protein is complete and folding takes place. All proteins are specially folded structures.
A Complicated Process
In humans, there are 20 amino acids. Most human proteins contain more than 100 amino acids that are folded into unique structures.
Over 100,000 different types of proteins are found in the body and each cell in the body contains 1 to 10 billion proteins. If you think about it, we have as many proteins in each of our bodies as there are stars in the universe.
Rare Disease
Some people are born with conditions in which they cannot create particular proteins. This leads to chronic problems. For example, some people cannot make the proteins that turn ammonia into urea. The urea cycle, an important metabolic cycle, is impaired.
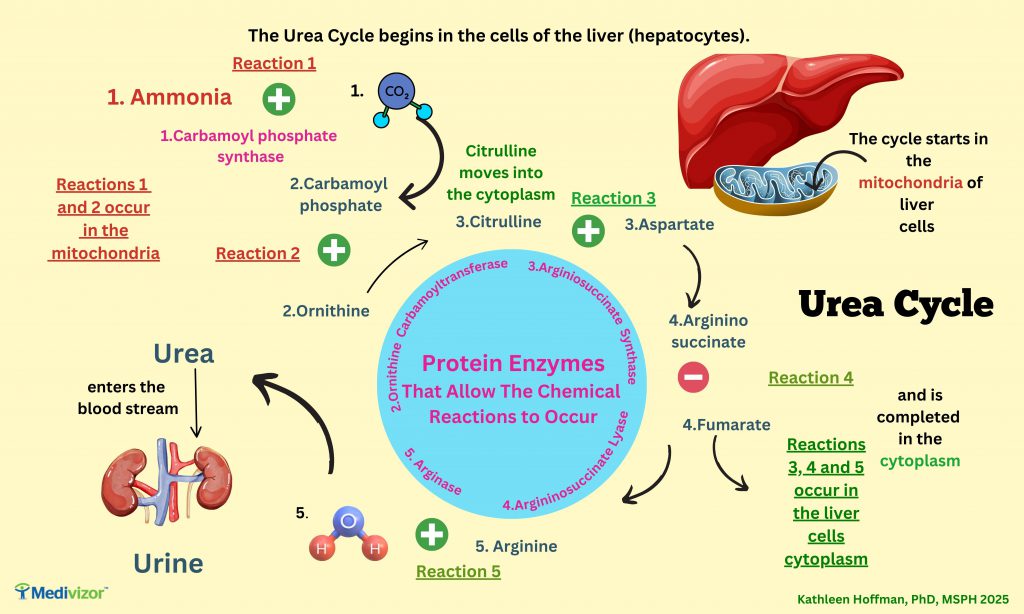
Ammonia is a waste product of digestion. It is a toxic substance that can kill cells, and is especially toxic to neurons. People with the rare disease, urea cycle disorders, cannot produce one or more of the proteins carbamoyle phosphate synthase, ornithine carbomoyltransferase, arginiosuccinate synthase, argininosuccinate lyases or arginase (the pink numbered proteins in the image above). These proteins are enzymes (ending with ase) which catalyze reactions—helping them to occur. [ 1 ]
Babies and adults with untreated urea cycle disorders experience a build up of ammonia which triggers vomiting, brain swelling, coma and death. Low protein diets with amino acid supplements and medications that can help control ammonia are available. But urea cycle disorders are “rare diseases with an often fatal course despite protein-restricted dietary management and pharmacological treatments. Liver transplantation remains the only curative treatment to date; therefore, there exists a significant unmet clinical need of novel therapies.”[ 2 ]
Novel Therapies: Therapeutic RNA to Treat Rare Diseases
One of the newest treatments being developed involves RNA interventions. Some of these genomic medicine interventions would replace the missing RNA that codes the proteins that humans need for survival. Others would suppress or even eliminate genes that code incorrectly. There are around 20 RNA treatments approved by the FDA to date.
There have been several challenges to using RNA as a treatment. One of the major issues is that RNA is fragile, degrading easily. If it were injected into the body alone, it would be destroyed immediately. Therefore, RNA needs a delivery system and a way to target the specific cells it needs to reach (targeting).
Lipid NanoParticles called LNPs have been developed to deliver RNA. The word nanoparticle gives a clue as to their size, they are tiny: between 10 nanometers and 1000 nanometers (nm) in diameter.
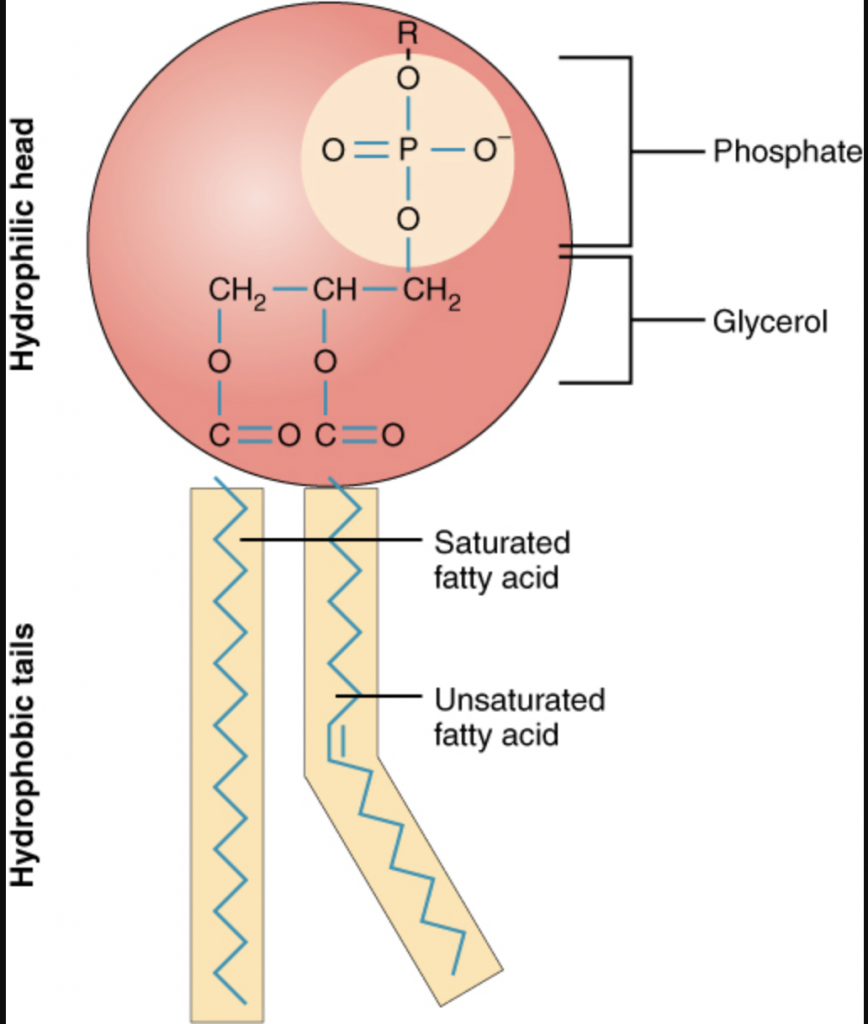
Lipid molecules or fats are interesting. They are made up of a phosphate head that is hydrophilic (attracted to water) and saturated and unsaturated fatty acid tails that are hydrophobic (turn away from water).
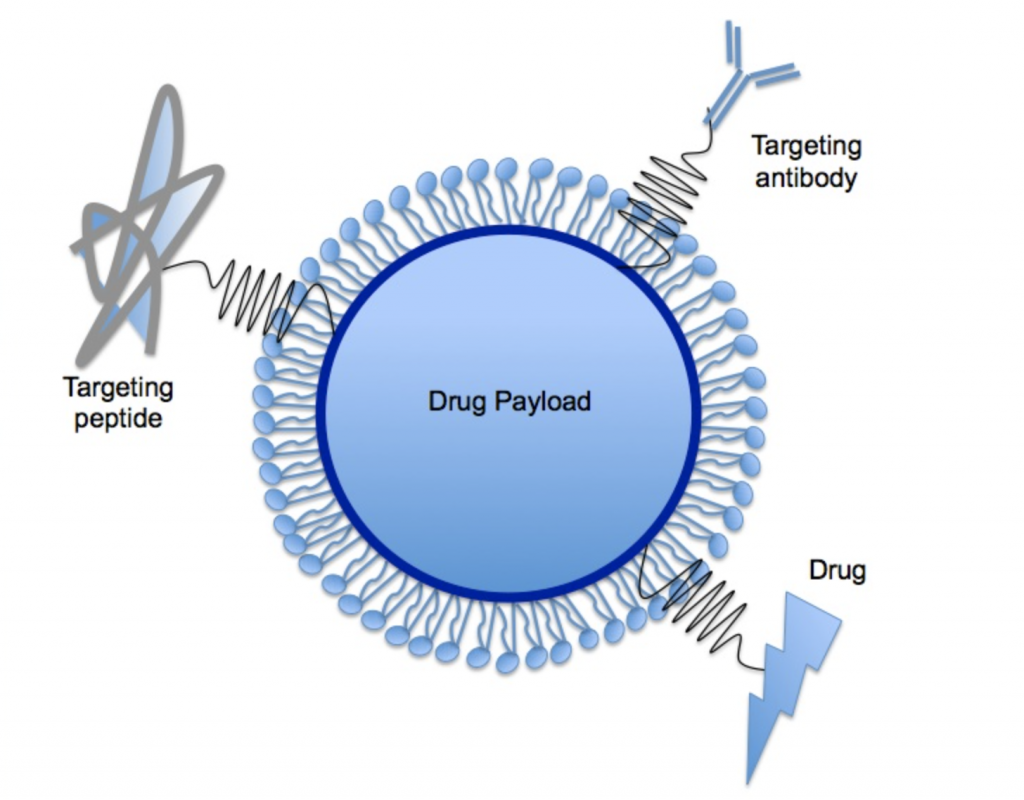
If you’ve ever placed oil in water you have seen the process of the formation of globules of oil in water. Lipids naturally create “balls” in water, with their heads facing out and tails facing in. These features make them ideal for the new task of holding a “drug payload” like RNA.
See the image below.
from https://commons.wikimedia.org/
wiki/File:SolidLipidNanoparticle.jpg
Of course, these systems are more complex than lipids surrounding the RNA. According to Precision Nanosystems, a company that produces these particles, there are four components within the “drug payload” which include:
1) hydrophilic stabilizer lipids which regulate the time these LNPs are in the bloodstream,
2) helper phospholipids that are involved in stability and helping the RNA escape from the LNP,
3) cholesterol which helps bring the LNP into the cell and release its payload (called endocytosis) and
4) cationic ionizable lipids which protect the nucleic acids and facilitate their transport in the cytoplasm of the cell. [ 3 ]
As can be seen in the image above, molecules that target the cells, like hepatocytes in the liver, can be attached to the LNP: targeting peptides, antibodies or drugs.
mRNA and Antisense oligonucleotides (ASOs) are two “drug payloads” that are being evaluated. ASOs “are short, synthetic, single-stranded oligodeoxynucleotides that can alter RNA and reduce, restore, or modify protein expression through several distinct mechanisms.” [ 4 ]Biotechs and pharmaceutical companies around the globe continue to research, including conducting clinical trials, that will provide real treatment for rare diseases like urea cycle disorders.
It is truly amazing to see how a few complex molecules can arrange and re-arrange to allow so many interacting functions to take place in service of making us live. Turning this knowledge to the purpose of new therapies for rare disease that push medical boundaries is at least as good.
References
[ 1] Barmore W, Azad F, Stone WL. Physiology, Urea Cycle. [Updated 2023 May 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK513323/
[ 2 ]Richard, Eva, et al. (2024). “Exploring RNA Therapeutics for Urea Cycle Disorders.” Journal of Inherited Metabolic Disease, vol. 47, no. 6, pp. 1269-1277, https://doi.org/10.1002/jimd.12807. Accessed 26 Feb. 2025.
[ 3 ] Ionizable Lipids to Optimize RNA-LNP Delivery. Precision Nanosystems. (2024). https://youtu.be/49ASLuxOSIY?si=0-wNqsaxrPh7kf9r Accessed 27 Feb. 2025.
[ 4 ]Rinaldi, C., Wood, M. (2018). Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14, 9–21 https://doi.org/10.1038/nrneurol.2017.148 Accessed 28 Feb. 2025.