There are people whose illnesses and symptoms have not been believed by medicine. Research has begun to change their experiences.
images/org/health/articles/mast-cell-activation-syndrome
Newly recognized disorder
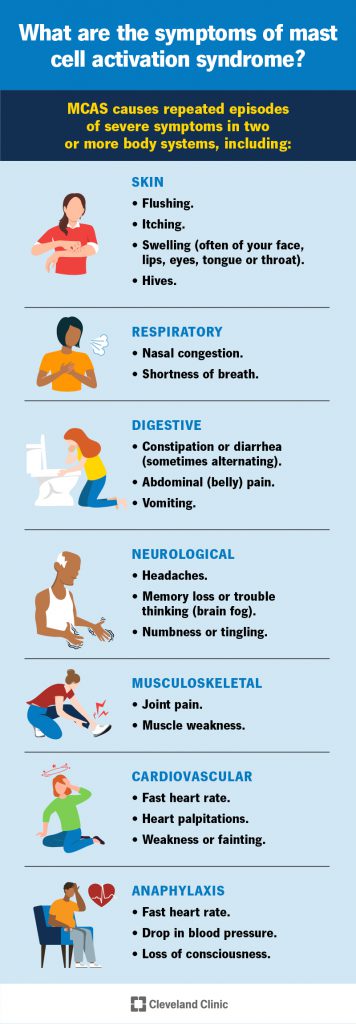
Mast Cell Activation Syndrome (MCAS) is a relatively new disorder (recognized just over 10 years ago). Symptoms of the condition are shown in this infographic by Cleveland Clinic. According to the American Academy of Allergy, Asthma and Immunology, “MCAS is a condition in which the patient experiences repeated episodes of the symptoms of anaphylaxis – allergic symptoms such as hives, swelling, low blood pressure, difficulty breathing and severe diarrhea. High levels of mast cell mediators are released during those episodes.”
Recent research connects this condition with hypermobile Ehlers-Danlos Syndrome (hEDS): demonstrating a high prevalence of MCAS in hEDS. [2] Ehlers-Danlos Syndrome (EDS) is a group of conditions with many variations in which hypermobile Ehlers-Danlos Syndrome is one. It is an inherited condition affecting connective tissue. Some of the most visible symptoms are stretchy skin, easy bruising, noticeable, stretched scars, and hypermobile joints. But since connective tissue is found everywhere in your body, from your blood vessels, skin and joints to your brain, stomach, intestines and other organs, there are other less visible symptoms. hEDS is the most common form of EDS.[2]
Studies have indicated that patients with hEDS and MCAS have higher incidence of recurrent infections. [2]
Genetic research to the rescue
Recently, research led by Dr. Michael Holick with Drs. Purusha and Arash Shirvani of Boston University School of Medicine has worked on decoding the genetic basis of MCAS and the increased infection risk in people with hypermobile Ehlers-Danlos Syndrome. A research team conducted whole-genome sequencing (WGS) on 18 participants with h-EDS. The study’s control group used seven first-degree relatives. [2]
The genetic changes found through the sequencing relate to dysfunctional mast cell activity, slowed immune system responsiveness and changes in the normal infection protective measures of the body.
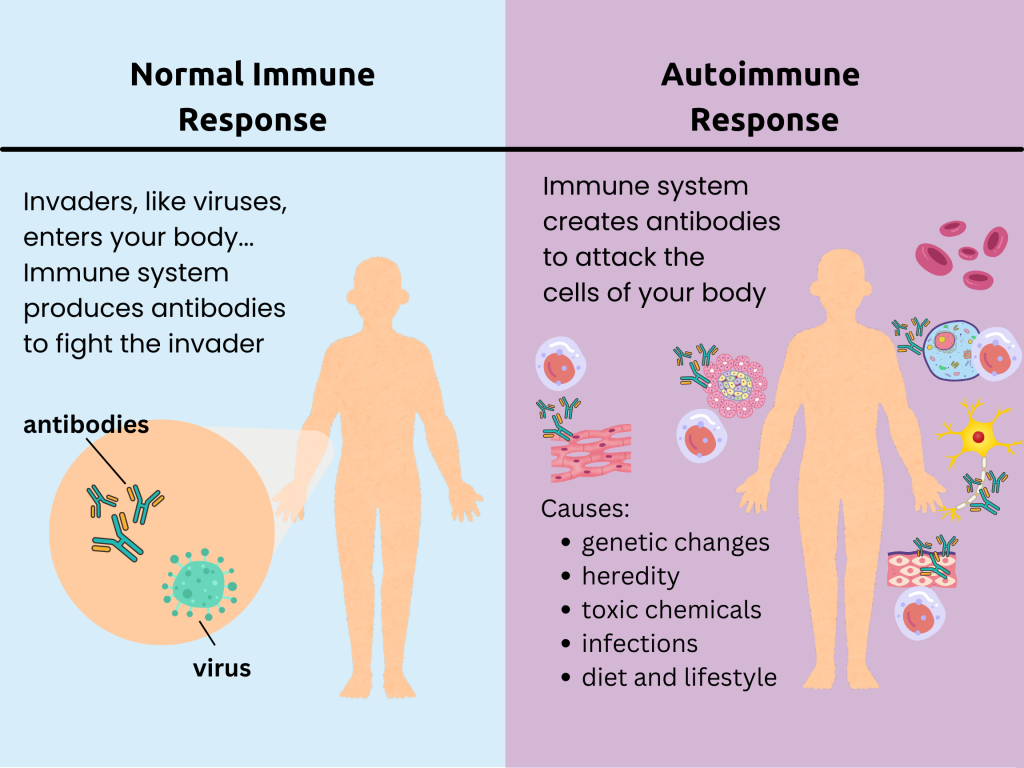
For example, changes were found in both HLA-B and the HLA-DRB1 gene. These genes each make a protein that helps the immune system distinguish between foreign particles from viruses and bacteria and the person’s own proteins. Autoimmune diseases, where the body’s immune system attacks itself—like rheumatoid arthritis—are caused by problems distinguishing between self and foreign objects.
In fact, this study found a novel variant in the HLA-B gene in more than half of the hEDS patients that was absent in the control group. Because HLA-B is part of the process of antigen presentation to immune cells (antigens are foreign substances), any changes in that protein could compromise the ability of the immune system to recognize and respond to germs. The participants in the research responded to survey questions about the frequency of getting sick and having recurrent infections: 61% of the hEDS patients reported that they had recurrent infection compared to 28.5% of the control group. [2]
Another genetic mutation discovered was in the HTT gene. The protein produced with instruction by the HTT gene participates in cytokine production in mast cells. Cytokines are communication chemicals released by mast cells to activate other immune cells. Changes in that protein can interfere with normal mast cell function. [2]
A third genetic mutation that the study found in these patients affected the gene that creates proteins in mucus, MUC1. Cells in the respiratory, gastric and reproductive tract contain this gene. The protein produced by this gene helps with keeping cells together (adhesion), with cells mobility, and with cell survival. This protein is also involved in kidney formation. A change in this protein can impact the production of the mucus barriers so important for keeping out infectious germs. [2]
One of the genetic mutations discovered in the participants with h-EDS was in the gene MT-CYB. When working properly, this gene—inside the mitochondria— instructs in making a protein that is called cytochrome b. This protein is part of an important group of proteins that helps in creating ATP, the energy source for all the cells in the body. [2] Without sufficient energy (ATP), normal cellular, tissue, and organ functions throughout the body slow down or must rely on less efficient energy production processes.
Other genetic variations found in this study were in genes ZNF521, ZNF717, and RUNX2, all of which are involved in bone development. One of the functions of mast cells has to do with bone development and bone breakdown. Many people with hEDS experience bone fragility. Another genetic variant discovered by this study was in MMP16. This gene is involved in collagen building and rebuilding. Changes in this can affect connective tissue. [2]
Being believed
So here is where the first part of the story, the part about sick people not being believed, happens.
This research is significant in explaining why people with these two invisible conditions are so negatively impacted by them. To begin with, genetic research shows that these poorly understood conditions are real. This directly contradicts many patients’ experience of gaslighting and disbelief in their symptoms.
In 2016, I wrote about a young woman with hypermobile Ehlers-Danlos Syndrome, POTS, and MCAS named Jessica Jacobs, who died at the age of 29. According to her friend, Whitney Zatzkin, Jess received threatening letters from clinicians, refusing her care. Whitney stated in her remembrance of Jess, “I stood there while clinicians, including some I deeply admired, all checked a mental box for triage and passed her to a never-named ‘someone else.'” In 2024, there are still patients experiencing this kind of clinical neglect.
Now, however, studies on clinician-associated trauma experienced by EDS patients are being published. [3] Blogs and news outlets are also focusing on this dangerous problem. In the BBC article, “Woman tries to understand why her body is giving up,” “Ehlers-Danlos Support UK (EDS UK) said a lack of awareness of the varying Ehlers-Danlos conditions makes it difficult for many to secure a diagnosis and receive the appropriate support and treatment.” In EDS is not “all in the head” John Ferman, President of Chronic Pain Partners writes, “Statements like ‘it’s all in the head’ from those physicians or psychiatrists who wrongfully attribute many typical EDS symptoms or those of comorbid conditions to psychological causes are damaging and dangerous to our community.”
In “Revenge of the gaslit patients,” STAT News featured the work at the Medical College of South Carolina, where patients with EDS who are also genetic researchers announced a gene mutation discovery associated with EDS. This is a major breakthrough. So is the study presented here today.
Drs. Holick, Shirvani and Shirvani propose a larger study to validate these preliminary findings but they feel that this work in genetic identification of the connections between infection recurrence, MCAS, and hEDS can be used to find new treatments and improve the lives of these patients.
An important benefit of their work is validation.
References
1.Dettmer, P. Immune: A journey into the mysterious system that keeps you alive. Random House; 2021.
2. Shirvani P, Shirvani A, Holick MF. Decoding the Genetic Basis of Mast Cell Hypersensitivity and Infection Risk in Hypermobile Ehlers-Danlos Syndrome. Curr Issues Mol Biol. 2024 Oct 17;46(10):11613-11629. doi: 10.3390/cimb46100689. PMID: 39451569; PMCID: PMC11506785.
3. Colin M.E. Halverson, Heather L. Penwell, Clair A. Francomano, Clinician-associated traumatization from difficult medical encounters: Results from a qualitative interview study on the Ehlers-Danlos Syndromes,
SSM – Qualitative Research in Health, Volume 3, 2023, 100237, ISSN 2667-3215, https://doi.org/10.1016/j.ssmqr.2023.100237.