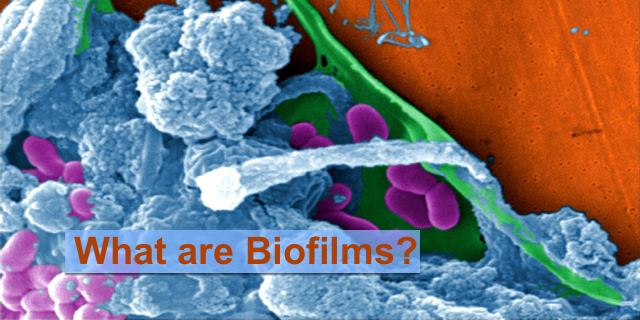
When bacteria are stressed, they stop living their lives singly, the way plankton do. Their adaptation is to find co-locate with other bacteria and build a type of fortress. That fortress is called biofilm.
Biofilm is an organized structure made of sugars (polysaccharides), proteins and DNA and of course, bacteria. In some cases other microbes like fungi, viruses and protozoa join the party. Building this fortress in stages, bacteria complete a complex environment where they can grow unchecked.
Stage one is called the reversible attachment stage and as the name suggests, it is a stage when biofilm is susceptible to antibiotics. Unfortunately, as the bacteria build the structure, they send microscopic extensions, called adhesions, that firmly attach the biofilm to the surface. This is the irreversible phase. During the next two phases, the bacterial cells become entrenched creating a fuller structure and signaling to other bacteria in the region to come and join their colony.
By this time, the bacteria have a nice safe place to live. Some leave their home to start their nomadic existence again but the biofilm community stays.
Now that you know the biofilm life cycle, you are probably guessing that biofilm is an enormous problem in healthcare. And you would be correct. Approximately 80 percent of recurrent or chronic infections have biofilms as their source. Recurrent urinary tract infections, periodontal disease, prostatitis are just a few of these. Biofilms can also cover indwelling medical devices like heart valves, joint replacement prostheses and catheters – even contact lenses, for example. And as previously noted, antibiotics are ineffective with these structures because the antibiotics can’t reach the bacteria. Biofilms on surfaces are also resistant to disinfectants.
Work to find ways to treat biofilms is ongoing. Nanoparticles have been studied as a delivery system to penetrate the structures and get antibiotics to the bacteria inside. Naturally occurring compounds from acacia trees, the Neem tree and actinomycetes (a type of bacteria) may also be effective against certain biofilms. The use of CRISPR technology is in the research pipeline.
Although biofilms are a nemesis in the field of healthcare, biofilms have been found to be beneficial. In fact, they have been used to convert pollutants to non hazardous substances and are used to cleanse waste water. They have even been used to improve plant growth. Science is beginning to get a handle on this unusual adaptation of bacteria, finding ways to treat harmful biofilms as well as effectively utilize helpful biofilms.
References:
- Muhammad, M. H., Idris, A. L., Fan, X., Guo, Y., Yu, Y., Jin, X., Qiu, J., Guan, X., & Huang, T. (2020). Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Frontiers in microbiology, 11, 928. https://doi.org/10.3389/fmicb.2020.00928
- Sharma, D., Misba, L. & Khan, A.U. Antibiotics versus biofilm: an emerging battleground in microbial communities. Antimicrob Resist Infect Control 8, 76 (2019). https://doi.org/10.1186/s13756-019-0533-3
Feature image: Photo by Janice Haney Carr, USCDCP on Pixnio